FDA reveals new requirements for breast cancer screening
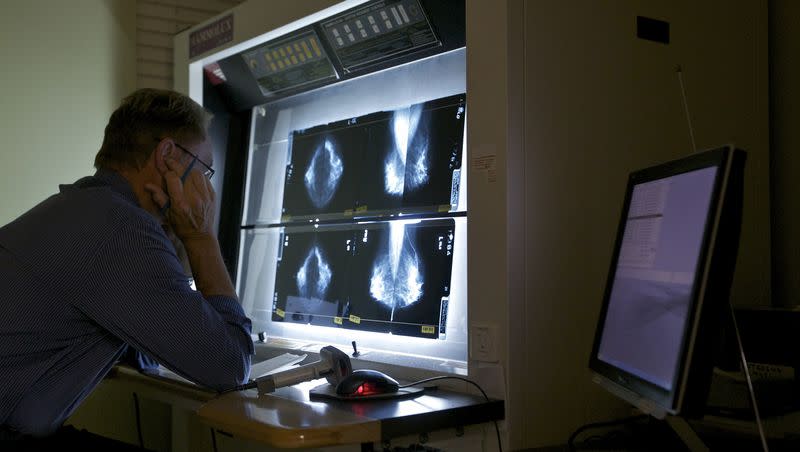
The Food and Drug Administration has announced new requirements for breast cancer screening that could help detect cancer earlier.
Here’s what we know.
Related
What happened: USA Today reported that the FDA’s new guidelines for breast cancer screening requires medical practices to “notify patients about the density of their breasts,” so that individuals with dense breasts know that this diagnosis “makes it harder to find breast cancer.”
ABC News reported while dense breast tissue “does not pose an immediate threat to health,” it is something for doctors to note and can heighten risk of breast cancer.
Related
What has been said: “Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” the FDA’s chief medical officer, Dr. Hilary Marston, said, according to NBC News. “Sometimes women that have breast cancers that are present, those breast cancers are not seen on the mammogram because they are hidden by breast density.”
Marston said that the FDA has been working towards advancements like these new guidelines and that “this means that more women have access to consistent, quality mammography. We remain committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
Related
Details: Medical providers are reportedly expected to begin adhering to the new requirements at some point in the next 18 months.
“It is so important at this point that women really go out, schedule your mammogram, get it done,” Marston said.