Amid opioid epidemic, Vivitrol finds success marketing to judges and jailers
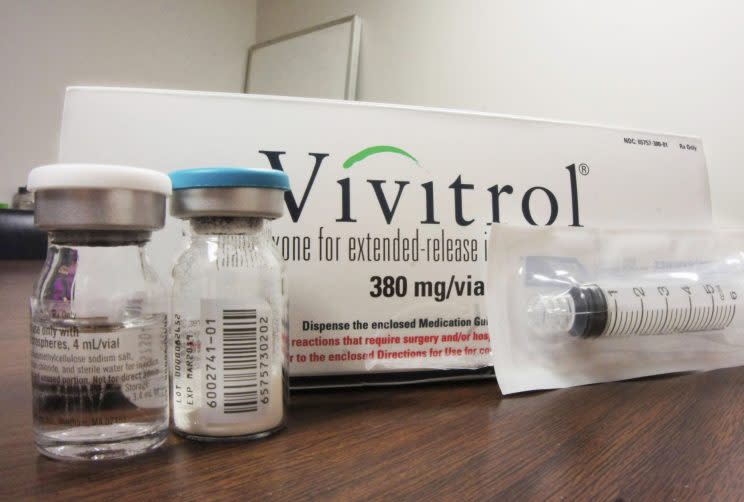
Alkermes’ marketing push led to Vivitrol’s growing acceptance as an opioid treatment in the criminal justice system — despite limited data on its effectiveness. Citing high dropout rates and higher risk of overdose, critics worry that jails and drug courts are now prioritizing its use over far better tested treatments.
Michael Prather, 55, an inmate at Hudson County jail in Kearny, N.J., has been using opioids since the age of 14. He had been free of heroin while serving an earlier sentence of more than 20 years, but after being released he relapsed, committed a crime, and wound up right back where he started.
“I still wanted that taste,” he said. “You know what I’m saying? I have tried other drugs to get away from that, but like I say, methadone and all was just another steppingstone to move on and onto another drug. It hasn’t done anything for me. Matter of fact, it is a drug itself. You know?”
Prather has heard of a new drug called Vivitrol. It’s an “antagonist” opioid-dependence medication that blocks the opiate receptors in the brain but does not promote the release of dopamine, the substance that activates the brain’s “reward system” and is associated with addiction. It’s an expensive ($1,100 a shot) monthly injection that requires patients to detox beforehand. Prather hopes this will get the monkey off his back.
“This is the best thing that ever happened, this Vivitrol situation,” he said. “You might try to do opiates just because you want that taste, but ultimately you will not be in a crisis situation when you wake up in the morning and you’re suffering from withdrawals and you need to get that drug.”
People outside the jail walls are now learning about Vivitrol thanks to an aggressive marketing campaign. Good-looking models stare blankly from posters on bus stops and billboards, asking, “What is Vivitrol?” It’s a simple question, but the answer is complex.
Second act: From bust to boom
The Food and Drug Administration (FDA) approved Vivitrol, the brand name for extended-release naltrexone, for treating alcohol addiction in 2006 and expanded the drug’s use to cover opioid dependence in 2010, based largely on a study conducted in Russia by Vivitrol’s maker.
There has not yet been a single completed study comparing Vivitrol with its competitors: methadone (Dolophine) and buprenorphine (Suboxone), which have long been considered the gold standards in opioid addiction treatment.

Alkermes, the Waltham, Mass., biotech company behind the drug, expected to have a hit on its hands after winning approval. But sales were slow at first.
Andrew Kolodny, the co-director of opioid policy research at Brandeis University, said physicians weren’t terribly interested in Vivitrol. He said there was scant data on its effectiveness, and clinicians were already having success with methadone and buprenorphine.
According to the American Society of Addiction Medicine (ASAM), methadone is an “opiate agonist” that activates opioid receptors in the brain. Buprenorphine is a partial agonist, meaning it activates the brain’s opioid receptors, but to a lesser extent. Both block the effects of other opioids, suppress withdrawal and reduce cravings.
Alkermes CEO Richard Pops adopted the unique approach of marketing the drug to officials in the criminal justice system, where opioids fueled cycles of incarceration for people like Prather.
“Vivitrol was not doing very well in the market until they came up with this strategy of targeting judges and lawmakers,” said Adriane Fugh-Berman, a professor of pharmacology at Georgetown University Medical Center.
“They really changed the playbook on this one,” Kolodny said. “The company turned its sales force on drug courts, judges and county executives. As a selling point, they are playing off the stigma of medicines like buprenorphine and methadone in settings where there might be a bias against them, exaggerating the downsides.”
When asked to respond to claims that Alkermes was disparaging competing treatments, company spokesman Matthew Henson said, “Our sales force is only trained and authorized to educate on Vivitrol in a manner consistent with [the] FDA-approved label.”

Approximately 90 people make up Vivitrol’s sales force, according to last year’s fiscal report.
Henson said the company doesn’t “assign reps to drug courts, prisons or any criminal justice environments.” Instead, he said, the sales reps “respond to where there is interest and need for Vivitrol.”
And they apparently won’t be slowing down anytime soon. The careers section of Alkermes’ website says the company is “rapidly expanding” its field sales group, noting “untapped market potential.”
In terms of sales, its strategy has paid off. The company’s annual reports have shown Vivitrol sales rising steadily. From 2015 to 2016, gross sales of Vivitrol increased by 70 percent, reflecting a 66 percent increase in the number of units sold and a 3 percent hike in prices.
In the first quarter of 2017, sales of Vivitrol reached $58.5 million. That’s a 33.4 percent increase over its $43.8 million in sales for the same period in 2016.
James M. Frates, the chief financial officer at Alkermes, said during an April conference call on earnings for the first quarter of 2017 that the company projects net sales for Vivitrol of $280 million to $300 million for the entire year.

“The underlying fundamentals of Vivitrol’s growth and potential are strong,” Frates told investors. “Since our year-end results call in February, we’ve seen a number of states expand from approximately 400 programs in 36 states to nearly 450 programs in 39 states. The robust growth of these programs, which range from public health initiatives to criminal justice programs in drug courts, prisons and jails, represents a leading indicator of the sustained growth of Vivitrol.”
During an earnings call in July 2016, Frates said Alkermes was “continuing to see accelerating sales growth” that was “driven by our focus on criminal justice programs.”
Criminal justice system
According to Henson, Alkermes is aware of about 200 prisons in the U.S. that incorporate Vivitrol into a broader program in which inmates with opioid dependence are often given the opportunity of a lighter sentence if they agree to treatment.
Alkermes provides inmates the first shot of Vivitrol for free, and the Affordable Care Act’s (ACA) expansion of Medicaid helps cover the high costs for the remainder of their reintegration after leaving prison. Methadone or buprenorphine are both much cheaper.
Before the ACA became law, if you were convicted of a distribution charge in New Jersey, you were banned for life from receiving Medicaid or cash benefits. If you were convicted of a possession charge with the same variables, you had to complete a residential treatment program to receive Medicaid. But the obvious problem is that you need Medicaid or health insurance to get into a program like that in the first place. The ACA eliminated those requirements.
Officials at the Hudson County jail’s reintegration program allowed Yahoo News inside the facility to see it firsthand. The staff took pride in emphasizing rehabilitation rather than simply incarceration. This is perhaps best symbolized by its recent name change from the Hudson County Correctional Facility to the Hudson County Corrections and Rehabilitation Center.
Frank Mazza, the program director, said it incorporated Vivitrol in its treatment regimens at the end of 2016 and that therapists conduct a risk-needs assessment to determine eligibility in the program: “You need to have a history of a mental health disorder, an addictions issue, and you have to be motivated towards change.”

He said patients get five days’ worth of Klonopin to help combat withdrawal symptoms during detox in the infirmary before they receive their first Vivitrol injection and leave the jail — so that they are released “completely drug-free.”
“If jails can be given a tool — granted, an imperfect tool, and I don’t think you’ll ever have a perfect tool — then jails should explore that option. They owe that to their population, to explore what has the potential to work,” Mazza said.
But inmates are not offered methadone or buprenorphine.
“I would say there’s a handful of jails in the nation that will bring methadone in. Nobody’s going to bring Suboxone in,” Mazza said. “We’re not bringing Suboxone or methadone in for one reason: It has street value. If we bring it in here and start giving it, it’s going to create a drug trade. It’s going to cause a ton of problems.”
There is overwhelming evidence that medication-assisted treatment (MAT) is preferable to no drug at all when handling opioid addiction.
Mazza said a tremendous problem is aligning jail treatment with community treatment. Most clinicians outside of jail tend to prefer methadone or buprenorphine because they’ve been proven to work. In short, jails are more comfortable with Vivitrol, whereas physicians are more comfortable with the older medications.
“So now we have a treatment regimen in place,” Mazza said. “You have to continue that treatment regimen. You [physicians] sort of have to abandon your approach to opiate addiction because of where this person came from. The jail has to take the lead here, and they have to continue that treatment and move off of where they’re comfortable.”

According to Mazza, the community health care network has to understand that a jail is a health care facility and is part of the network because of its role in fighting the heroin epidemic.
Physicians push back
A study published in March 2016 in the New England Journal of Medicine found that the rate of relapse among convicted criminals with opioid addiction who received Vivitrol was lower than those without medication-assisted treatment. It was conducted in part because of what the paper’s authors called Vivitrol’s growing “acceptance in the criminal justice system despite limited data on effectiveness.”
When asked about these findings, Corey Waller, the senior medical director for education and policy at the Camden (N.J.) Coalition of Healthcare Providers, said, “One, MAT is better than abstinence, no matter which medication. Two, generally post-incarceration also has probation attached, which can alter outcomes. Three, naltrexone is a great medication when used on the right patient. The issue is not that it is a bad med — it is not — it is that it may be the wrong medication for most patients as a first-line therapy. So putting barriers up for access to the other medications based on stigma rather than science is the issue.”
Kolodny said the addiction specialists resistant to abandoning methadone or buprenorphine do not necessarily think Vivitrol is a bad treatment. In fact, he pointed out, many would like to see more research done so they can determine which patients would benefit from it the most.
Fugh-Berman said Vivitrol should not be presented as the only option or a superior option to the opioid medications that have a long and successful history of treating addiction.
“Is it a useful medication for some people? Yes. Has it been tested against proven effective medication? No,” she said bluntly.
Alkermes promoted a study conducted in Russia with 250 patients to secure FDA approval. Over the course of six months, nearly half the participants who received Vivitrol failed to stay off opioids, but it was more effective than a placebo.
Why did it conduct the study in Russia when there are so many addicts in the U.S. anyway? When posed this question, Henson said that when this phase-three trial began, there was a “large population of untreated patients with opioid dependence in Russia without access to buprenorphine or methadone, which were widely available in the U.S. at that time.”
Potential for Vivitrol in new addicts
Brandeis University’s Kolodny believes Vivitrol might be an appropriate treatment for young patients who haven’t been using for long. A physician might reasonably want him or her to stay off methadone and buprenorphine; those regimens may need to be kept up for years or they could create a dependence of their own.
But Kolodny said Alkermes’ marketing strategy has resulted in the aggressive use of Vivitrol in what he considers a bad population for the drug: moderate to severe opioid users, including heroin injectors.
“Patients for whom we know abstinence-based approaches — which Vivitrol basically is — are very risky is exactly where the drug is being promoted most aggressively,” he said.
Deni Carise, a clinical psychologist and the chief clinical officer at Recovery Centers of America, made a similar assessment. She found Vivitrol particularly helpful for young adults who had recently become addicted to opioids and wanted to quit drugs altogether.

“These are folks where I’d hate to see them try a medication opioid replacement therapy like methadone or buprenorphine if they have a very short career of drug use.”
The Camden coalition’s Waller agrees that Vivitrol can be an effective treatment when patients are appropriately chosen, but worries that Vivitrol’s sales force is denigrating other effective medications.
“It’s just that when it’s being pushed in courts and at the state level, and in a lot of different areas and in legislatures as well, it’s being pushed as better than or equivalent to methadone or buprenorphine. That’s just wrong,” Waller said. “I mean, that’s just inaccurate. And we don’t have those data to show that it’s equivalent to or superior to those. Yet they’re pushing it as if it’s equivalent in outcomes to those, and we just don’t have the data to say that. That’s my biggest issue.”
Waller found success prescribing Vivitrol to patients in the past but fears some of its use in the criminal justice system amounts to “practicing medicine without a license.” He said it takes a great deal of medical expertise, specialty training and evaluation to determine who might benefit from which medication.
“The reality is that you have a subset of people who are vulnerable to suggestive thought because they want to do the right thing and they don’t know how to do it,” he said.
Derek’s story
Derek Larson, 33, of Spanish Fork, Utah, credits Vivitrol with saving his life. He became addicted to pain pills after tearing his rotator cuff while pitching for the University of Utah’s baseball team when he was 19. When his prescription ran out, a friend recommended he start smoking heroin because it’s cheaper. His grade-point average plummeted and he quit the baseball team — forfeiting a full-ride athletic scholarship.
“It was heroin from there on out. I went on an eight-year binge. I did things unimaginable,” Larson told Yahoo News.
He was prescribed Suboxone, but it didn’t help, mainly because he’d get it cheap through insurance and sell it to other addicts at a profit to buy heroin. He’d take Suboxone as a substitute only if he ran out. A two-month stint in rehab only seemed to get him more drug connections.

“I would steal so much money. I’m surprised I’m not dead, honestly,” Larson said. “I never did shoot it up, thank goodness. I had so much money to keep smoking. Most people have to turn to shooting it up.”
Larson finally got clean for six months by taking Vivitrol shortly after its FDA approval for opioid addiction. But he convinced his family he was in the clear and stopped taking the shot — so he could do heroin again.
“I went on another three-year spree. I had a few DUIs, drug possession. The DEA [Drug Enforcement Agency] bust down my front door because they thought I was dealing, which I was a little bit to my friends,” he said. “Coming from a great family, I was just in this pit. I was like, ‘This is my life now.’ I didn’t think I could have a life after this.”
Larson went to jail for about six months. When he got out, he was busted for drug possession and landed back in jail. At that point, three years ago, he started using Vivitrol again and hasn’t stopped. He said he’s been clean since. He’s recommended Vivitrol to five people from his hometown.
“It’s worked for me and don’t care to get off of it,” he said. “I think I could do good now, but I know myself. I’ve never been able to. So why get off of it? Why take that chance? It’s not hurting me.”
Risk of overdose
Despite its potential benefits, Vivitrol (like all drugs) comes with dangers. In this case, overdose is a primary concern — not from Vivitrol itself, but from opioids by patients who relapse.
It’s long been known that when a heroin addict comes out of detox, his tolerance returns to normal, and if he then started using the same amount of heroin as before, he could easily die of an overdose. With naltrexone exposure, patients don’t just return to normal — they become hypersensitive to opioids.
Kolodny said if someone’s opiate receptors are blocked, the brain responds by producing more of them, a process called “up regulation.” This is not a problem if a patient keeps taking naltrexone because those extra receptors are also being blocked. But if a patient stops taking the medication, he or she would become more sensitive to opiates.
“If you’re more sensitive to opiates, you can more easily die of an overdose when exposed to opiates,” Kolodny said.
A study on chronic naltrexone use in rats published in the scientific journal Brain Research found that blocking opiate receptors made them more sensitive to morphine. The authors wrote, “These results demonstrate that chronic opiate receptor blockade increases the number of receptor sites for morphine and that this increase in receptors is accompanied by a neuronal supersensitivity … to morphine.”
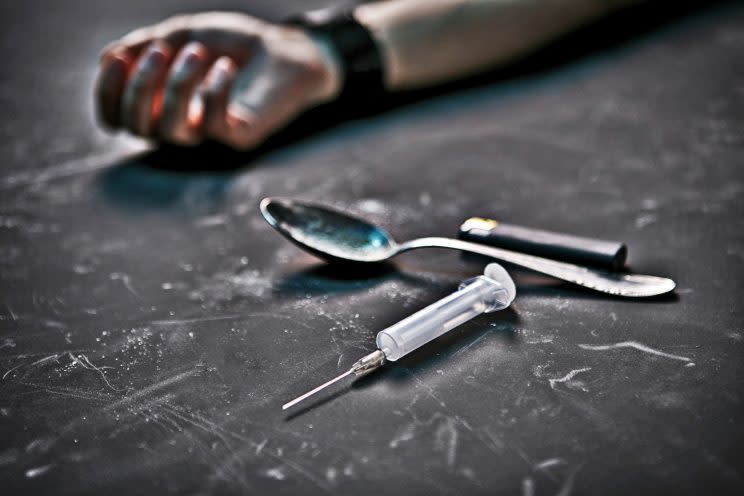
Kolodny said this is “very likely to be the mechanism” for the increased risk of death. Others agreed with Kolodny’s assessment.
“Naltrexone significantly reverses tolerance, abstinence moderately reverses tolerance, and opioid replacement only mildly reverses tolerance,” Waller said. “So naltrexone, if used in patients who are not motivated, and as compared to other treatment modalities, significantly increases the chance of overdose.”
A recent study in the Journal of Substance Abuse Treatment looked at a western Massachusetts county jail with a Vivitrol treatment program similar to Hudson County’s. The researchers looked at 67 inmates with opioid addiction who were given extended-release naltrexone — 47 started treatment before leaving jail, and 20 started at outpatient treatment centers after leaving.
Four weeks into the study, the retention rate was 55 percent for patients who started in jail and 25 percent for patients who started after leaving. Six months into the study, the vast majority had given up on Vivitrol: only 21 percent and 15 percent, respectively, had stuck with it.
Three patients died from overdose three to five months after release.
“The treatment attrition and striking rate of overdose deaths are concerning, and support expanded availability of opioid agonist treatments prior to release and other evidence-based supports and retention strategies in the community,” the study’s authors wrote.
The FDA package insert for Vivitrol addresses the potential for increased risk of overdose in naltrexone-treated patients.
Alkermes acknowledges this “risk of opioid overdose” in its list of serious side effects. Aside from the aforementioned sensitivity, trying to override the blocking effect by taking large amounts of opioids is another concern.
Oscar Aviles, the assistant county administrator for Hudson County and one of the reintegration program’s chief architects, said he is doubtful that former inmates would keep using heroin if they weren’t getting the effect because most live below the poverty line.
“These folks steal to get high. I don’t see them pumping heroin into their system when they’re not getting the benefit out of it,” Aviles said. “I understand the overdose issue, but once these folks put a bag of dope in themselves and they’re not going to get high, I don’t see them going back to buy another bag.”
VICTORY study
Alkermes conducted a study of 403 patients called “Vivitrol’s Cost and Treatment Outcomes Registry” (VICTORY) in 2012 and 2013. It has been registered on clinicaltrials.gov.
Alkermes has not yet published its findings in full, but the company did present some information at an ASAM meeting in 2014 that it also provided to Kolodny. He said it appears from the presentation that Alkermes tried to “spin the data” to make Vivitrol look good, but it’s plain to see that “the results were awful” if you “read between the lines.” In short, he said, this Vivitrol study showed a troublingly high dropout rate.
One slide in the presentation lists 14 reasons why participants stopped taking the drug. According to Kolodny, only two reasons indicated successful discontinuations, and these accounted for 8.5 percent of participants: “patient feels treatment goal met” and “physician intended planned course of treatment met.” All the other reasons, Kolodny said, should be considered treatment failures. These include “lost to follow up,” “withdrawal by patient,” “study terminated by sponsor,” “lack of efficacy by patient,” “noncompliance,” “incarcerated” and “death.”
Five patients died during the study. Three of those five were overdoses.

“They went through this slide pretty quickly, I’d imagine. You kind of have to read between the lines to get a sense that this was a total failure,” Kolodny said.
Elsewhere, the slides do show that patients who received at least six consecutive months of Vivitrol shots did well. But Kolodny said more than half the patients had dropped off by that point.
Kolodny said he’s very frustrated that Alkermes has not yet published the results of the study in full because opioid addiction is America’s most urgent public health problem.
“You would think in the midst of a public health crisis, if they’ve got data that’s helpful for people to know — for example, which patients shouldn’t be getting that drug and which patients should — they should be sharing that data.”
Kolodny thinks that Alkermes does not want to publish the data because the subset of patients who should be taking Vivitrol is very small, and the company wants to see it prescribed broadly.
When asked about the VICTORY study’s results, Henson, the Alkermes spokesman, said a manuscript with data from it is undergoing peer review for publication consideration. He said this “real-world, observational” study was different from a “placebo-controlled clinical study” in many respects: There was no control group, treatment length was not pre-specified, prescriptions were filled through commercial channels, patients who dropped out could enroll again and treatment planning (which was decided by prescribers) was not consistent among patients.
Marketing and lobbying
Alkermes assumed the responsibility of marketing Vivitrol from an outside company, Cephalon, in December 2008. According to Alkermes’ 2012 annual fiscal report, “Vivitrol is the first commercial product for which we have had sole responsibility for commercialization, including but not limited to sales, marketing, distribution and reimbursement-related activities. We are increasingly focused on maintaining rights to commercialize our leading product candidates in certain markets.”
In September 2016, Alkermes revealed at an event for investors that it had more than 300 programs in 35 states at that time aimed at incorporating Vivitrol into state policy. Each program targets one of four categories: criminal justice reentry initiatives, drug courts, public health agencies and the state laws themselves.
According to the Center for Responsive Politics, Alkermes spent $4.4 million in lobbying last year and $4 million in 2015.
Waller said Alkermes is pressuring lawmakers in states such as Pennsylvania and Ohio to pass legislation that would increase access to Vivitrol and limit it to other medications. “It’s talked about pretty openly,” he said.
Fugh-Berman directs Georgetown’s PharmedOut research and education project, which studies the effect that pharmaceutical marketing has on prescribing practices. She said pharmaceutical companies will advertise to whoever can increase market share — physicians, patients or someone else.
She said it’s not unusual for the companies to push for the passage of laws that benefit their drugs. Still, she was astonished that lobbyists actually got legislators to write the name of the drug into a bill under consideration in Indiana.
One version of the law read: “Vivitrol or a similar substance may be required to treat opioid or alcohol addiction as a condition of parole, probation, community corrections, pretrial diversion, or participation in a problem solving court.” The bill did not pass in that form.
“It’s creative. It’s unusual. It raises a lot of questions and ethical concerns,” she said. “Here is a company that is talking down treatments that have been proven to be effective in treating opioid addiction.”
Stigma
Kolodny said the big-picture problem is that people are dying in the U.S. from a treatable condition.
“So we have medicines that will help keep people alive and treat this condition that’s become an epidemic in the United States, [but] inadequate access to these treatments,” he said. “Yet you have many people reaching for — and in some cases being referred for by a court — to a treatment that is untested in many ways.”
According to critics, Alkermes has capitalized on the stigma surrounding methadone by deftly incorporating it into sales pitches — reaching high levels in the U.S. government.
In May, shortly after visiting the Vivitrol manufacturing facility in Wilmington, Ohio, Health and Human Services Secretary Tom Price criticized methadone and buprenorphine as “just substituting one opioid for another.”
This outraged more than 700 specialists who signed a letter expressing “grave concern” that Price ignored scientific evidence and contributed to a stigma surrounding effective treatments.
“The perception that persons receiving long-term therapy with medications — especially buprenorphine and methadone — are not actually in recovery is widespread but grossly inaccurate,” the letter reads.
Access to all treatments
Terrence D. Walton, the chief operating officer of the National Association of Drug Court Professionals, said the practice his office advocates is that drug court participants have access to all three of the evidence-based interventions that the FDA has approved to treat addiction — to the extent a program can do that.
“They are required to participate in treatment, but the decision as to which particular combination of treatment interventions is something that’s recommended by the treatment provider,” Walton said.
Ben Nordstrom, the chief clinical officer of Phoenix House and Phoenix Life Centers in Brooklyn, incorporates Vivitrol into his practice, but said there isn’t enough scientific data to determine what the best medication is for each patient. He informs patients of the relative advantages and disadvantages of each so they can make a decision together.

“There have not been head-to-head comparisons where we can say, ‘This is the best kind of medication for this type of patient, whereas this is the right medication for this kind of patient,’ so what we’re left with is a mix between what the patient wants and we can advise, but we can’t dictate what someone can do,” Nordstrom said. “My perspective as a physician is that they’ve made an informed choice and they’re getting what they want.”
As a result, he said, the choice of an appropriate drug for any patient comes down to an “ideological rather than a scientific position”: Does the patient prefer something like buprenorphine to prevent any residual withdrawal symptoms? Does the patient want to be off anything that could be habit-forming?
“From my perspective, the only wrong medication choice for someone is no medication,” Nordstrom said. “Ultimately, the right medication for any one person is the medication that they want and that they’re going to take and use in a way that will help them engage with the psychosocial services and the other treatments.”
When asked about Vivitrol’s marketing push, Nordstrom said, “Most drug manufacturers really kind of market aggressively, so I don’t know if it would be any different.”
The scourge of heroin
The leading cause of accidental death in the United States is drug overdose — and the opioid epidemic is driving it. In 2015, there were 20,101 overdose deaths related to prescription pain relievers and 12,990 related to heroin, according to ASAM.
The Centers for Disease Control and Prevention estimates that 91 Americans die from an opioid overdose every day, and that more than a half-million died from drug overdoses from 2000 to 2015.
Another inmate at the Hudson County jail, Mavis Travis, 59, of Jersey City, has been fighting heroin addiction since she was 19. She said the last time she used the drug was about a year ago.

She said her past attempts at drug rehabilitation programs have been unsuccessful because she didn’t participate wholeheartedly. Travis said she participated “just to get out of here,” gesturing at the jail around her.
“For any program, if you’re not going in there wholeheartedly, it’s not going to work,” she said. “But if you got in your heart that you’re not going to do nothing, you’re not going to do it. You can sit in a room full of people getting high and it’s not going to affect you. The program comes from your heart. It doesn’t come from a piece of paper or a 12-step.”
Travis has a newborn grandson she hasn’t gotten to see yet.
“I haven’t smelled his little breath, counted his little toes or nothing,” she said. “So that’s my mission right there, to set an example for him. You have to lead by example, so it’s about that time.”
Travis looked around at the other women incarcerated at Hudson County and noted that she’s much older than most of them.
“I’m too old for this. There’s nothing here but a bunch of kids. These are kids. I think I’m about the oldest one in here,” she said. “I know I am.”
Travis has heard of Vivitrol but hasn’t tried it.
Another inmate at Hudson Country, a 54-year-old man with an opioid addiction, said he’s willing to give Vivitrol a shot, but has never tried methadone or buprenorphine.
He said a guest speaker who came to his jail tier piqued his interest in Vivitrol.
“He sounded very confident about the drug,” he said. “I’m at the age where I don’t want to go back out there and use. I’ve heard a lot of good things about Vivitrol.”
“I’m tired of all the people in my neighborhood that are dying of opiates,” he said. “We need something strong and positive to rebuild on. I’m glad they came out with this new drug.”

Read more from Yahoo News:


