FDA authorizes the first at-home rapid COVID-19 test. Here's what you need to know.

In a major development Wednesday, the Food and Drug Administration announced that it has issued emergency use authorization (EUA) for an at-home, rapid COVID-19 test made by Lucira Health. The California-based company describes the product as an “all-in-one-test kit” intended for home use for people 14 and older who have been suspected by a doctor of being infected with COVID-19.
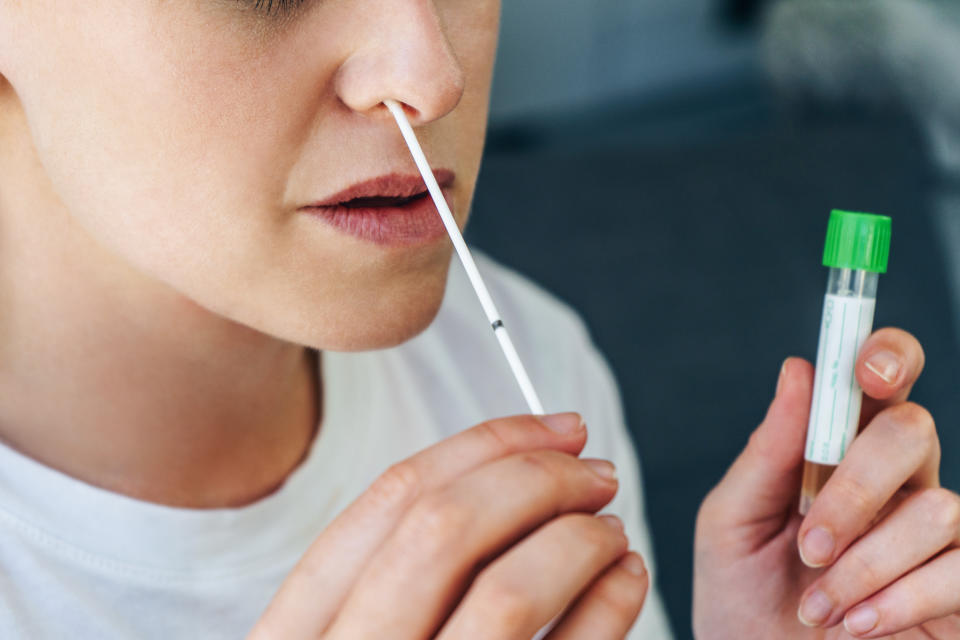
The test would be available by prescription from a health care professional, and would not be available for purchase over-the-counter.
In a statement, FDA Commissioner Stephen Hahn outlined the significance of the approval. “While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” Hahn said. “This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission.”
The news comes as COVID-19 cases continue to trend upwards in at least 46 states, with reports of many Americans waiting in lines as long as five hours to receive tests. Dr. Amesh Adalja, senior scholar at the Johns Hopkins Center for Health Security, tells Yahoo Life that it’s unequivocally good news. “I think it's the first step down a pathway that hopefully will allow us to do rapid tests at home on a daily basis — and be able to know our status and kind of move through this pandemic in a much better way.”
To further clarify the significance of this breakthrough, here’s what you need to know.
It is intended for at-home use but can be administered by a doctor for those 13 and under
Lucira’s test is designed for those 14 and above who have received a prescription from a doctor for a COVID-19 test based on a suspected case. But the company has also received approval for it to be administered by a medical professional to those under 13. The test is specifically designed for those experiencing symptoms of the virus, not those who are asymptomatic.
The test requires a self-administered nasal swab and provides results within 30 minutes
Much like Pixel, LabCorp’s at-home COVID-19 test, which received an emergency use authorization from the FDA earlier this year, Lucira’s requires that individuals self-administer a nasal swab (rolling the swab around five times in each nostril). But while Pixel then asks individuals to drop it into a vial and mail it to a lab, Lucira instructs users to drop it into a vial, stir it 15 times, snap it closed and then attach it to a small plastic test unit.
Within 30 minutes, the light-up test unit will flash either a negative or positive result — but the company notes that “a positive test result can be generated in as few as 11 minutes.”
It uses what’s known as “LAMP” testing, and is more effective than antigen tests
In a document outlining the FDA authorization, the company describes it as a “molecular in-vitro diagnostic test” which “extracts genetic material from the virus and amplifies it.” This type of testing — sometimes known as LAMP (loop-mediated isothermal amplification) — has been employed by other companies during this pandemic, but Lucira is the first to create an at-home version.
A rapid LAMP test is markedly different from the current most popular rapid tests known as antigen tests. Antigen tests detect a protein linked to SARS-CoV-2, which means that are best at “identifying a person who is at or near peak infection.” Molecular tests like Lucira’s more closely resemble the current gold standard for COVID-19 tests, polymerase chain reaction, or PCR. Both tests rely on detecting genetic indicators associated with the virus, meaning they can detect low levels of the virus.
It may not be as accurate as a PCR test, but is still extremely beneficial
Lucira’s test is faster than a PCR test, which typically takes between 48 and 72 hours, but Harvard University immunobiologist and New York Times science reporter Katherine Wu pointed out on Twitter Wednesday that this may not be a good thing. “LAMP testing is like a quick and dirty version of PCR-based testing, sort of. Both look for genetic material. Both are also signal amplifiers...” Wu wrote. “LAMP is generally cheaper, more convenient, less cumbersome than PCR. It can be done outside a lab. But it's also typically less accurate.”
Adalja doesn’t consider LAMP testing to be necessarily less accurate, and says Lucira’s test likely has more value. “I think it has comparable sensitivity and it is something that can be done much simpler, much cheaper and easier than a PCR because it doesn’t require some of the same technical steps that are required,” Adalja says. “Usually when you have a LAMP-type of test, that really lends itself to point of care because you can visually tell whether or not something is positive or negative versus more of the analytic stuff that has to be done to call a positive PCR test.”
The company predicts it will cost $50, and be ready in spring 2021
The company says that the test will soon be available to “patients served by Sutter Health in Northern California, and Cleveland Clinic Florida in Miami-Ft. Lauderdale” and that it hopes to be available to Americans nationwide by the spring of 2021. The test is expected to cost around $50.

For the latest coronavirus news and updates, follow along at https://news.yahoo.com/coronavirus. According to experts, people over 60 and those who are immunocompromised continue to be the most at risk. If you have questions, please reference the CDC’s and WHO’s resource guides.
Read more from Yahoo Life
Want lifestyle and wellness news delivered to your inbox? Sign up here for Yahoo Life’s newsletter.